Since making its debut in the 1970s to analyze radiocarbon (14C), accelerator mass spectrometry has significantly matured – but like all things in science, there is always room for more discoveries. In this post, we discuss how AMS techniques are being applied across a variety of interesting applications.
Accelerator mass spectrometry (AMS) is a particle accelerator technique that measures radioisotope ratios with high precision. Most AMS systems use a tandem electrostatic accelerator.
Here’s a brief overview of how accelerator mass spectrometry works, but check out this graphic for a more detailed explanation:
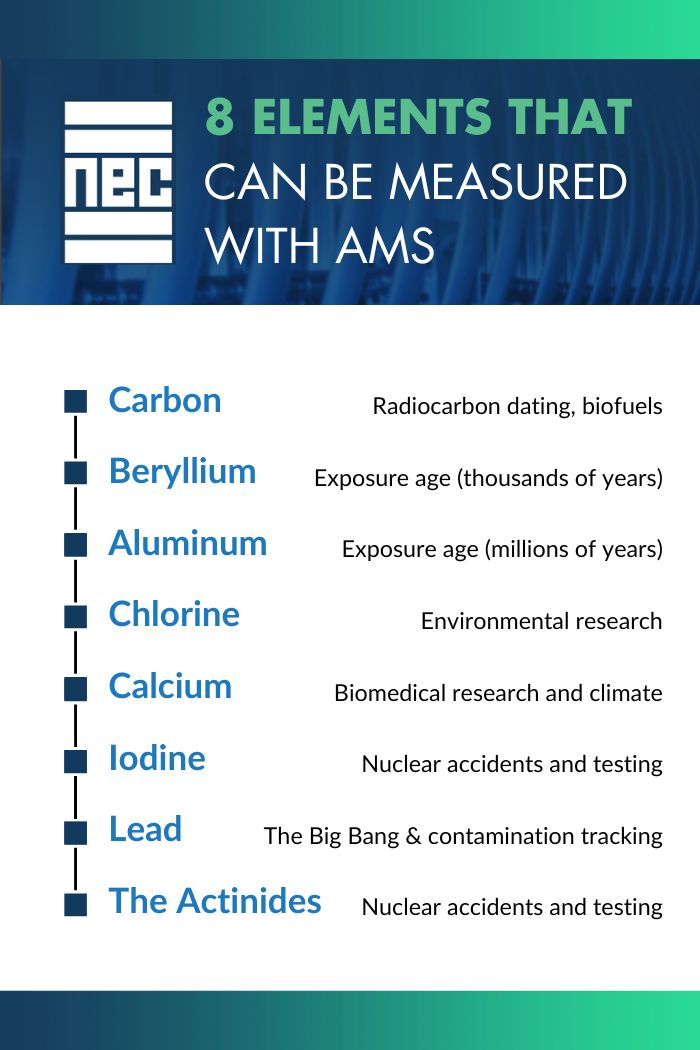
This technique is commonly used for radiocarbon dating with applications in archeology, oceanography, biomedicine, and more. However, there is growing worldwide interest in studying other elements, expanding AMS into many other areas of science. Here, we review eight AMS elements (or group of elements) that offer exciting potential.
Upwards of 155 labs across the world are using accelerator mass spectrometry to measure carbon.
Radiocarbon dating – the process of dating organic materials by measuring how much 14C is left in a sample after decaying over time – is just one (well-known) use case.
Carbon AMS presents other interesting opportunities in various aspects of environmental science. Today, interest in using carbon AMS to validate the purity of biofuels is growing. We’re also getting inquiries about using AMS to analyze the pollutants that come out of trash burning.
NEC has provided just under half of all AMS equipment around the world and continues to improve carbon AMS with every system we manufacture.
Just as organic materials absorb carbon, rocks and other sediment surfaces absorb beryllium. With AMS, scientists can measure the amount of atmospheric beryllium (10Be) in a sample to study the exposure age – that is, the time that has passed since rock surfaces were exposed to the atmosphere. This understanding also gives us a picture of what the composition of the atmosphere was like over two-hundred thousand years ago.
For example, the 500 kV Pelletron at Qingdao National Laboratory for Marine Science and Technology in China has measured beryllium samples with more precision than much higher voltage accelerators.
Similar to beryllium, aluminum (26Al) can be measured to study the exposure age of rocks and other sedimentary material – but on a much longer time scale. We’re talking millions of years old! This provides insights into landscape evolution, erosion, and tectonic activity.
For example, the group at IUAC in Delhi, India, has been measuring aluminum AMS for over a decade on their 500 kV Pelletron.
Chlorine AMS is an emerging element of focus with interest in environmental research. However, chlorine faces more isobaric interference than other elements, making it more difficult to detect. In fact, it used to be only measurable on an AMS system of 5 MV or higher. Today, researchers are optimizing systems to distinguish chlorine measurements with an AMS system of 1 MV or less.
The SUERC lab in Scotland is well known for chlorine measurements on their 5 MV Pelletron accelerator.
Calcium is an essential element of biological systems, and AMS can be used to study the turnover rates of calcium in bones and other tissues.
In addition to biomedical research applications, calcium AMS can be used to study past climate patterns, the sources and sinks of calcium through various Earth systems, and nucleosynthesis processes in stars and supernovae.
For example, the PRIME group at Purdue University in the United States measures calcium for cosmogenic and fission research.
Radioactive iodine is a byproduct of nuclear fission processes in nuclear reactors, accidents, and weapons testing that poses significant health risks to humans and the environment. Iodine AMS can be used to measure the iodine in the atmosphere to trace the dispersion of the element in the environment.
For example, after the 2011 nuclear meltdown in Japan following a devastating earthquake and tsunami, NEC’s AMS systems detected the plumes of iodine and other harmful materials in the air in Seattle just a few days later. This application is vital for environmental monitoring and protection.
For example, the group at Idaho National Labs in the United States routinely measures iodine from nuclear reactors on their custom 500 kV Pelletron.
Scientists have found lead all over Earth and on meteors in space that can be traced back to the Big Bang. With lead AMS, we can improve our understanding of the early universe and processes that occurred after the Big Bang.
Lead AMS also has an important environmental application, helping researchers identify and track sources of lead contamination.
For example, Australia National University uses a 14 MV large NEC vertical tandem to measure lead AMS and look back in time millions of years.
The actinides are a series of elements located at the bottom of the periodic table that are known for their radioactive properties, including plutonium (Pu) and uranium (U).
The most pressing application of actinide AMS is nuclear forensics and waste management. By analyzing samples from nuclear incidents, scientists can trace the origin of nuclear materials. Similar to the iodine application, monitoring actinide isotopes can provide insights into environmental contamination from nuclear facilities or weapons testing.
For example, the ANSTO group near Sydney, Australia, uses a 1 MV Pelletron accelerator with advanced actinide measurement capabilities to measure up to 8 isotopes at once.
Each of these elements of interest can be studied with accelerator mass spectrometry. To do so, you need an AMS system that’s optimized for different energies, depending on the isotopes being studied:
Ultimately, the higher energy the system, the easier it is to discriminate these elements and atomic masses.
To date, the focus is still on carbon and all of the applications carbon AMS has to offer – especially when it comes to environmental monitoring. Techniques for other isotopes are continually being developed, and as the technology continues to improve, AMS will become more accessible to scientists around the world.
With over 50 years of accelerator experience, NEC provides industry-leading ion beam accelerator systems and related components designed to expand the research goals of scientific communities around the world. Tell us about your research needs by contacting us today.